Transport et devenir en milieux poreux d’une communauté bactérienne issue de boues de stations d’épuration
Spreading of sludge from waste water treatment units on open fields is widely practiced in France and other countries. Such process may diminish the volume of pollutants throw back directly to natural water resources of rivers or lakes. However waste water plant effluents are rich in pathogenic microorganisms and other bacteria uncommon to soil environments (Dumontet 2001). As a result spreading of sludge can cause drinking water contamination or modification of the soil ecology by bacterial transport through aquifers. Coliforms are commonly found in waste water plants and some are often responsible for gastrointestinal infection outbreaks (Crockett 2007). Coliform bacteria are microorganisms that primarily originate from the intestines of warm-blooded animals but can survive in other environments. By testing for coliforms, especially the well known thermotolerant coliform Escherichia coli, one can determine if the water has probably been exposed to fecal contamination (Tian et al. 2002; Unc et Goss 2004). The WHO (World Health Organisation) recommends E. coli detection in water to track fecal contamination. Bacteria from sludge able to travel through soils can thus negatively impact human health and the environment if not under control. Bacterial transport studies usually focus on one type of bacterial strain instead of an entire bacterial community like in sludge (Abu-Lail et Camesano 2003; Bradford et al. 2006; Stevik et al. 1999). Most of such studies describe two main mechanisms that are responsible for preventing bacterial transport: filtration and adsorption (Stevik et al. 2004). Filtration typically involves the physical blocking of bacterial movement through small pores. Filtration depends on the cell to porous media grain size ratio (Bradford et al. 2006), water degree saturation (Smith 1985) or clogging (Rijnaarts et al. 1996). Adsorption involves the bacterial cells to be attracted towards the porous media grain surface and to stick on it. Many approaches have been used to explain bacterial adhesion on solid surfaces.
Among them the physicochemical approach which can be interpreted using the DLVO theory (van Loosdrecht 1989). Hydrophobic attraction and electrostatic repulsions have been reported to be major factors influencing bacterial adhesion (Li et Logan 2004; van Loosdrecht 1990). It has been widely admitted that most bacterial cells and sediments are typically negatively charged at groundwater low ionic strength and mild pH, resulting in electrostatic repulsions occurring between bacterial cells and soil particles(Choi et al. 2007). In such conditions bacterial transport is enhanced. Jacobs et al. showed that differences in transport behaviours through sand among various bacterial strains were partly due to the physicochemical cell surface properties (Jacobs et al. 2007). They concluded that small bacterial cells with hydrophilic and negatively charged surface characteristic are most likely to be transported on long distances. Despite the increasing number of bacterial transport related articles, the literature lacks any reference about the fate of bacterial sludge community in soil environments. Waste water plant sludge is known for housing riche bacterial communities suggesting all sorts of transport behaviours depending on each bacterial strain. In this paper we are interested in studying the transport and fate of bacterial sludge community through two porous media (sand and soil). SSCP was used to characterize the community before and after transport through the porous media. We also looked for the presence of thermo tolerant coliforms as it is a valuable tool used for tracking recent fecal contamination.
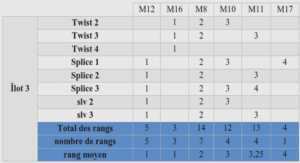
The experimental setup consisted in Plexiglas columns (Ø = 50mm, length = 150mm) filled either with sand or soil aggregates. Porosity and porous media characteristics are listed in table 1. Before use, the sand was thoroughly rinsed with milliQ water on a 40µm filter (VWR international, 11cm, type 417) then heat treated and oven dried for at least 2 hours at 120°C. For sand, the columns were filled under water. Before packing into the columns, soil aggregates were rewetted on a suction table at a water potential of -30 hPa. Next, the aggregates were compacted by layers of 3cm in the column. Last, these columns were vacuum saturated with sterile solutions. The bulk densities are given in table 1. Experiments were carried out with a sterile 10-4 mol.l-1 CaCl2 background solution. Upward circulation of the solution through the column was obtained by mean of a peristaltic pump Ismatec SA reglo digital MS-4 (Switzerland). Each column experiment was repeated two times with each sample of sludge. Three samples of sludge were obtained from a rural waste water plant (Caromb, south ofCaCl2 ). Each experiment lasted 90 minutes which corresponds to about 3 pore volumes of the porous media used. Controls consisted of the same column experiments as described above but without the sludge. For each experiment, enumeration and other molecular biological techniques were carried out on aliquots of the whole water recovered at the outlet of the columns.