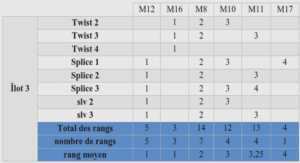
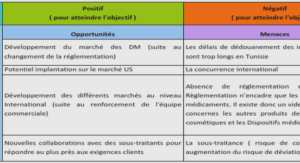
Preparation of the oxazolopyridinimines via silver catalysis
To a round-bottomed flask were added, in the following order, dimethylsulfoxide (0.8 M compared to pyridine), propargyl alcohol (1.2 equiv.) and difluoropyridine (1.0 equiv.). Sodium hydride (1.2 equiv.) was then added portionwise over 30 minutes. The reaction was then put under nitrogen and stirred at room temperature under nitrogen atmosphere until completion was indicated by TLC analysis. The excess reagents were then quenched with water. The mixture was extracted three times with diethyl ether. The organic phases were combined and washed with water and brine and then dried over magnesium sulfate. The solvent was evaporated under reduced pressure. The crude was then purified by silica gel chromatography (98 : 2 petroleum ether : ethyl acetate) The NMR tube was then heated to 60 °C in an oil bath. After 1H NMR showed no starting material remaining, the reaction mixture was filtered on a pad of silica and washed with ethyl acetate. The crude was then purified by silica gel chromatography (95 : 5 petroleum ether : ethyl acetate) leading to the pure compound. To a microwave vial (from 2 mL to 5 mL) were added 0.1 mmol of fluoropropynyloxypyridine, 0.11 mmol (1.1 equiv.) of aniline, 0.4 mmol (4.0 equiv.) of sodium carbonate, 2.5 mL of chloroform and 0.01 equiv. (10 mol%) of silver salt (AgNTf2). The reaction mixture was then filtered on a pad of silica and washed with ethyl acetate. The crude was then purified by silica gel chromatography (90 : 10 petroleum ether : ethyl acetate) leading to the pure compound.
To a microwave vial (from 2 mL to 5 mL) were added 0.1 mmol of fluoropropynyloxypyridine, 0.05 mL of water (27.5 equiv.) and 0.05 mL (12 equiv.) of acetonitrile, 2.5 mL of chloroform and 0.01 mmol (10 mol%) of silver salt (AgNTf2). The vial was then heated for 1 hour at 100 °C in the microwave. The reaction mixture was then filtered on a pad of silica washed with dichloromethane : methanol (95 : 5). The crude was then purified by silica gel chromatography (99 : 1 dichloromethane:methanol) leading to the pure compound. mixture and the tube was heated to reflux in an oil bath. The reaction is monitored by 1H NMR. When no starting material remains, the reaction mixture is filtered through a pad of silica with ethyl acetate as the eluent. The crude product was then purified by silica gel chromatography (60 : 40 petroleum ether : ethyl acetate) leading to the pure aldehyde (notably unstable).mixture and the tube was heated to reflux in an oil bath. The reaction is monitored by 1H NMR. When no starting material remains, the reaction mixture is filtered through a pad of silica with ethyl acetate as the eluent. The crude product was then purified by silica gel chromatography (60 : 40 petroleum ether : ethyl acetate) leading to the pure aldehyde (notably unstable). In a microwave vial (from 2 mL to 5 mL), 0.1 mmol of fluoropropynyloxypyridine was dissolved in 2.0 mL of toluene. The vial was sealed and heated in the microwave to 200 °C for 2 hours. The solvent was then evaporated and the product purified by silica gel chromatography using petroleum ether : ethyl acetate as the eluent (90 : 10).