Microstructure and Magnetic
Abstract
Ni-P alloys were prepared by electrodeposition on a copper substrate at different plating conditions (applied potential and deposition time). The morphology, composition, microstructure, structure, and magnetic properties of the films were investigated by scanning electron microscopy, energy-dispersive spectrometry, X-ray diffraction, and vibrating sample magnetometry, respectively. The phosphorous content is about 8.6–12.2 at.%. The deposits exhibit either a nanocomposite structure where Ni(P)–1 and Ni(P)–2 solid solutions are embedded into an amorphous matrix, or a mixture of Ni2P phosphide and Ni(P)–1 and Ni(P)–2 solid solutions. The coercivity and magnetization are plating condition dependent. All samples exhibit a soft magnetic character with coercivity lower than 58 Oe. The squareness ratio Mr/Ms values that are in the range 0.019– 0.123 correspond to a multidomain (< 0.1) for all samples except sample B (−1.15 V, 10 min) which falls into a pseudo-single domain (Mr/Ms = 0.123).
Introduction
Electrodeposition has received much interest since it is an effective method to produce dense and thick deposits over a large surface area in a relatively short time by means of a simple process. It is also a low-temperature processing route to produce nanostructured materials. The increased interest in technology for electrodeposition is due to its low cost, versatility, high production rates, and industrial applicability [1–3]. Nickel-phosphorous (Ni-P) alloys have attracted many considerations owing to their good mechanical and chemical properties such as high hardness, high strength, high corrosion resistance, good wear resistance, solder ability, and uniformity of coating thickness [4]. Due to their high hardness, Ni-P alloys are suitable for diamond turning applications including fabrication of large optics and other high-precision parts [5]. Moreover, Ni-P coatings can be used as catalytic coatings for hydrogen evolution reactions [6], thin-film resistors, and under-layer in thin-film memory disks [7, 8]. The Ni-P system is very complex and exciting over a wide range of concentrations since the crystalline compounds Ni12P5, Ni5P2, Ni2P, Ni3P, NiP2, and NiP have different structures which are P content dependent. Also, Ni5P2, Ni2P, and Ni3P phosphides have attracted much attention due to their excellent properties and potential applications such as corrosion-resistant, oxidation-resistant luminescent devices and electronic components [9]. It has been reported that nanocrystalline (NC) and amorphous phases with different P contents are formed and the average grain size decreases when the deposition content of P increases in the Ni matrix [10]. The amount of P in the Ni-P alloys influences the microstructure and the characteristics of the as-deposited films [11]. The transition from a crystalline to an amorphous structure takes place over a range Author’s personal copy J Supercond Nov Magn of P concentrations rather than in an abrupt way at a certain composition [12–14]. The Ni-P alloys are generally classified into three categories: low P (2–7 at.%), medium P (8–18 at.%), and high P (more than 19 at.% P) [15, 16].
Materials and Methods
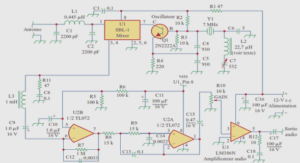
Ni-P films were electrodeposited under potentiostatic conditions onto polished copper substrates with an exposed area of about 1 cm2 and a thickness of about 60 μm. Three applied potentials (−1, −1.15, and −1.3 V) were set up in order to follow the formation mechanism of the Ni-P deposits during 10 and 20 min. Hence, six deposits named samples A (−1 V, 10 min), B (−1.15 V, 10 min), C (−1.3 V, 10 min), D (−1 V, 20 min), E (−1.15 V, 20 min), and F (−1.3 V, 20 min) were prepared. The experiments were performed using an electrochemical technique by means of the three-electrode potentiostat/galvanostat PARSTAT 2253. The substrates were mechanically polished with SiC emery paper and subsequently treated in order to remove the impurities by two successive baths—acetone (99.5 %) then ethanol (95 %)—and finally rinsed in deionized water. The pre-cleaned copper substrates were used as working electrodes. A nickel foil was used as a counter electrode and saturated calomel KCl (SCE) as a reference electrode. The distance between the anode and the cathode was maintained at 4 cm in order to ensure uniform deposition. The operating temperature was 70 ± 2 ◦C and the bath pH was maintained at 3–4. The plating parameters such as the bath composition and the operating parameters are summarized in Table 1. Microstructural analysis was performed on the surface and cross section of the Ni-P deposits by scanning electron microscopy (SEM, Zeiss DSM-960A) operating at a voltage of 20 kV with energy-dispersive X-ray spectroscopy (EDX). Structural changes of the deposited coatings as a function of the applied potential and deposition time were studied by X-ray diffraction (XRD) using a PanAnalytical powder diffractometer in Bragg Brentano (θ − 2θ) geometry in the range 20◦–120◦ with a step size of 0.02◦, a counting time of 10 s/step, and a Cu-Kα radiation (λCu = 0.154056 nm). The XRD profiles were analyzed through the Maud program [18] which is based on the Rietveld method [19]. Instrumental parameters, like 2θ correction, peak asymmetry, and peak broadening parameters (U, V , W) of a LaB6 standard sample, assumed to have no size and strain broadening, have been used as fitting parameters in the software. The quality of the refinement is estimated from the various numerical criteria of fit, namely the weighed residual factor (Rwp), the Bragg factor (Rb), the expected factor (Rexp), and the goodness of fit (Gof) obtained from the analysis procedure. Magnetic measurements were carried out using a vibrating sample magnetometer (VSM, MicroSence). The measurements were conducted on the as-deposited samples by applying an external field of 20 kOe in the plane and out of the plane.