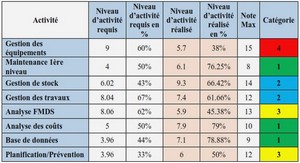
Hydrogen Storage Properties
The physical and chemical properties of materials related with safety and design of hydrogen storage system decides its suitability for prevalent use as hydrogen store. The cost of material, synthesizing processes, raw elements and their abundance are vital practical considerations. However, the primary scientific aspects related to hydrogen sorption characteristics of alloy are,
a. Storage capacity (gravimetric, volumetric, reversible)
b. Thermodynamic properties (Enthalpy of adsorption, absorption and
decomposition)
c. Kinetics (hydrogen absorption, desorption)
d. Ease of activation
e. Cyclic performance
f. Gaseous impurity resistance
Based on literature review, the following important aspects are discussed in detail. For hydrogen st orage, it is essential to study various storage capacities, the absorption kinetics and the behaviour when first time exposed to hydrogen. The necessity of activation could be decided from behaviour of storage material when first time exposed to hydrogen storage .
Storage capacity
Gravimetrie storage eapacity
The gravimetric capacity is the weight percentage of amount of hydrogen absorbed in metal. The theoretical gravimetric capacity of BCC alloys is around 4 wt%. The BCC alloys have much less hydrogen capacity compared to the Mg based hydrides (7.6 wt.%) and complex hydrides (more than lOwt.%) [10]. The light weight metals like Sodium,Lithium and Beryllium are also hydrogen absorbing metals. This class of hydrides is popularly known as chemical hydrides. The well-known complex hydride LiBH4 has capacity of 18wt% [10]. Though the gravimetric capacities of Mg based and light weight metal hydrides have been reported on higher side compared with BCC alloys, they have kinetics and thermodynamics limitations. They decompose at high tempe rature and are difficult to dehydrogenate. The BCC alloys can usually absorb hydrogen at room temperature under relatively low pressure of 1-2 MPa [17-19].
Reversible storage capacity
The reversible hydrogen capacity is an amount of hydrogen desorbed out of hydrogen absorbed in metal system during repetitive cycles of absorption and desorption under normal conditions. It is measured with in terms of reversibility of the system. The reversibility is defined as percentage amount of desorbed hydrogen out of absorbed hydrogen. This is also one of the important parameters evaluated for hydrogen storage systems. Miraglia et. al. reported reversible capacity of2wt% in case ofTi-V-Cr system at 288K . The hydrogen was desorbed by reducing the pressure from 1 MPa to 0.02 MPa step by step. Okada et al. reported the reversible hydrogen capacity of2.2 wt% for as cast Ti-35V-xCr(x=37,43) at 313 K [22].
Kinetics
The kinetics of hydrogen absorption and desorption is a result of the way in which overall transition from metal to metal hydride take place. For metal hydrides, the hydrogenation process takes place ln four steps physisorption, chemisorption, nuc\ei formation and growth.
a. Physisorption: When hydrogen molecules become weakly immobilized on a metal surface, due to Van-der-Waals or dipole interactions, they are said to be physically adsorbed, or physiosorbed. The enthalpies of physisorption are typically less than 0-5 kJ/mole [24].
b. Chemisorption: Chemisorption starts with breaking of bond between hydrogen atoms in molecules and forming strong bond between hydrogen and alloy atoms. In this way hydrogen atoms get absorbed inside metal/alloy with initiation of hydride phase. The range of energy involved in chemical absorption is reported to be around 20-150 kJ/mole [24] ofH2 which is very high as compared with physisorption.
c. Nuc/ei formation and growth: The initiation of metal hydride phase is called Nuclei formation. The nucleation is usually at the surface defects/strain fields/grain boundaries. Nuclei start to grow on metal surface or inside metal/alloy [24].
These ail steps collectively determine the kinetics of hydrogen absorption. The step with slowest kinetics limits the overall kinetics of phase transformation. The slower step is known as the rate limiting step. It is a crucial part of study to find out the rate limiting step, which can further help to find out the ways to overcome the rate limiting step.
First hydrogenation
Sorne environmental adsorbates surrounding external surfaces of solid [26] could contaminate the fresh and clean surfaces exposed to environment before hydrogenation. It forms a layer of oxides/ corrosion! hydroxyl. This layer passivates the surfaces for hydrogenation and acts as a barrier for hydrogen to be absorbed within the metal, is called a Surface Passivation Layer (SPL) [27]. The hydrogen absorption!desorption processes are delayed due to SPL originated from contaminants and remnants of solvents in alloys produced using wet chemistry [27 28]. The waiting duration before start of hydrogen absorption is known as incubation time. The incubation could be of few minutes, few hours or few days. The longer incubations are detrimental; thus, a pre-treatment is needed. Generally, the contaminants and remnants are extracted by keeping the sample under vacuum at high temperatures [29]. Samples can also be cleaned with purging of an inert gas used to remove pre-adsorbed species [29]. This removal process is popularly known as degassing and can be supervised by weight loss during degassing (gravimetric system) or decrease in the pressure (volumetric system) [30].
The presence of SPL mostly affects the first hydrogenation and once SPL removed the hydrogenation cycles carried out after first hydrogenation shows quicker absorption. Thus, an activation process is required to remove SPL. Activation is the process of breaking SPL barrier to absorb hydrogen quickly in metal host using high temperature and/or pressure by exposing it to hydrogen first time [27]. For industrial applications and for on-board hydrogen storages, working at high temperature and pressure is not desirable. The high temperature and pressure could raise the safety issues. The limits for working temperature and pressure are set by US DOE as 353K- 393 K and 2 MPa – 4 MPa respectively [31]. It indicates that activation is additional time-consuming process at additional cost. Thus, avoiding activation is beneficial and many researchers have been working on reduction in incubation time as well as first hydrogenation at reduced temperature and pressure. In the current work, we have developed understanding about the parameters affecting the activation and its mechanism and have worked towards complete elimination of activation or achieving the first hydrogenation at room temperature under low pressure.
Generally, the mechanism of activation could be explained as follow,
• When alloy exposed to hydrogen first time, hydrogen faces difficulty for physisorption due to surface passivation layer on alloy particles. In order to reach alloy surface, hydrogen molecules supposed to break the passivation layer and then get adsorbed. First time the task of physisorption may be more difficult than for the further cycles.
• Chemisorption could proceed after a physisorption of H2 molecules over the surface of metal host mostly at cracks/surface defects/grain boundaries. This may initiate the formation of metal hydride phase.
• The growth of hydride phase could create un-uniform strain fields In metal particles.
• High stress and strain fields may be developed at concentrated areas like cracks/surface defects/grain boundaries which leads to fragmentation of metal particles.
• The fragmentation of metal particles may lead to exposure of fresh and c1ean metal host surface for hydrogenation. The available surface area for hydrogenation also increased with reduced particle size due to fragmentation.
• The enlarged fresh and c1ean metal ho st surface could absorb hydrogen quickly. An instantaneous change in kinetics of hydrogen absorption may end up with absorption to full hydrogen capacity.
1. INTRODUCTION |