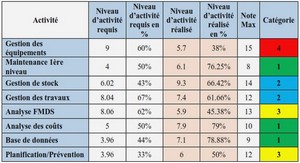
COORDINENCES NON USUELLES DU GERMANIUM
Divalent germanium and tin species
L2GeS (L2 = PhNC(Me)CHC(Me)NPh, S = Cl, I, Me, OMe).
Introduction
The divalent species of Group 14 elements are the heavier carbene analogues; for recent reviews, see Refs 6-10. They are generally transient species. As a general rule, when these species contain unfunctionalized organic ligands, they undergo rapid oligomerization and polymerization. In the last two decades, two principal methods of stabilization have been particularly investigated. Thus, various kinetically and/ or thermodynamically stabilized divalent germanium and tin compounds have isolated in a monomeric state. The use of bulky groups bound to the group fourteen element to prevent aggregation permitted the first syntheses of stable monomer divalent species; the most noticeable are the dialkyl-germylene and -stannylene reported by Lappert and coworkers, 12 the first stable monomeric arylgermylene ((Mes* )2Ge (Mes* = 2,4,6- tBu3- phenyl)) described by Du Mont and co-workers,14 and the well known aryl-germylene and -stannylene Tbt(Tip)M14 (M14 = Ge, Sn; Tbt = 2,4,6-tris[bis(trimethylsilyl)methyl]- phenyl, Tip = 2,4,6- i Pr3-phenyl) of Okazaki and co-workers. 25, 29 The ligand backbone may also play an important role in improving stability. Thus, the presence of ligands with donor side arms on the germanium or the tin element can (by transfer of electron density) reduce the deficit on the central atom. Many different stable species have been prepared by taking advantage of this approach. For example, the compound reported in 1997 by Meller and co-workers 37 is, to our knowledge, the first example of an intramolecular base stabilized homoleptic dialkylgermylene. Various amino-, phosphino-, thioalkoxy-, alkoxy- and aryloxydivalent species have also been isolated in monomeric form owing to this method of stabilization; one should mention in particular the works of Jutzi and co- I. Composés du germanium et étain II stabilisés par le ligand ß-diiminate. 12 workers, Veith and co-workers, 15,18,40,66 Parkin and co-workers, 67 Jurkschat and co-workers, Dias and co-workers 31,35,42,69,80,81 and Roesky and co-workers.2-5 Althrough a large number of homoleptic divalent germanium and tin species have been isolated using these concepts of stabilization, there are fewer examples of heteroleptic compounds having been reported, thought the first functional heteroleptic germylenes R(Cl)Ge (R = Et, Ph) were obtained as viscous oils in the seventies by our group.6 Few solid state structures of such heteroleptic compounds have been described. Noteworthy examples are in the area of thermodynamic stabilization, the acetylacetonatogermylene R(I)Ge (R = OC(Me)CHC(Me)O) obtained by Stobart 4 in 1979 being the first heteroleptic germylene structurally characterized. One should also mention the germylenes and stannylenes described by Veith and co-workers 6 and Lappert and co-workers 8 in 1988. After 1998, various other structures with, in particular aminotroponiminate, amidinate ligands have been reported; the research groups of Dias,1,31,35,69,80,81 Richeson, 53 Lappert,22,43,45 Jutzi,28,32,54,71 Jurkschat,77,78 Roesky 2-5 and Fillipou 46,68 have been particularly involved in this work. In the area of kinetic stabilization, the main results concern the compounds obtained by Power and coworkers 50 using bulky aryl groups. For our part, we have recently described divalent germanium and tin compounds supported by salen60-62 or amine-substituted alcoholates. 57-59,65 Our interest has since turned to the study of heteroleptic germanium(II) and tin(II) species supported by the chelating ß-diketiminato ligand L2
Synthesis, characterisation and structures
The synthesis of the halodivalent compounds L 2 (X)M (M = Ge, Sn) is very easy (Eqn (1)), since the reaction of the ß-diketiminato lithium with the dihalodivalent species in toluene or ether affords the corresponding germylenes and stannylenes in quantitative yields. (1) M14 = Ge, Sn; X = Cl, . . + BuLi N N Ph Ph Me Me M N X N Ph Ph Me Me Li N N Ph Ph Me Me H MX2 -LiX, 25°C Et2O -BuH, -78°C The methyl, methoxy and, the trimethylstannyl homologuess were isolated by metathesis reactions (Eqn (2)). (2) N N Ph Ph Me Me Ge X . . + RM toluene -MX, -78°C N N Ph Ph Me Me Ge R . . M =Li, R = Me, Et, OMe Na, R = SnMe3 All the L2 (R)Ge compounds are soluble in aromatic solvents, but the halide analogs L2 (X)Ge show a lower solubility (specially the iodo compound) in these solvents probably as a result of a more ionic structure. All these compounds have been fully characterized (Table 1). Table 1 : Selected 1H and 13C{1H} NMR (C6D6) data for L2 (S)Ge I. Composés du germanium et étain II stabilisés par le ligand ß-diiminate.It is noteworthy that all the 1H and 13C NMR signals due to the ligand appear slightly downfield from the corresponding signals in the free ligand, in particular the methine resonances. The 119Sn NMR spectrum of the tin compound exhibits a broad resonance at – 280 ppm, indicating that tin in this compound is basically threecoordinated in solution. For the iodogermylene, the 1H and 13C NMR (table 1) chemical shifts are more downfield than those in the other compounds; this may be a result of an increased positive charge on the germanium (or on the ligand backbone) due to a weakly coordinated iodide anion (L2Ge¼ I). The chemical shifts of the halogeno compounds vary strongly with the solvent, with polar solvents leading to strong deshielding probably as a result of a solvent polarization of the germanium¼ halogen contact. For the halogeno divalent species the mass spectra show in all cases that the base peak corresponds to the L2Ge+ cation resulting of a loss of halogen. The low degree of ion pairing in the iodo compound is revealed in the gas phase by the absence of the molecular ion peak in the mass spectrum. It is noteworthy that the cationic ligand germanium(II) L’2Ge+ species (L’2 = ArNC(Me)CHC(Me)NAr, Ar = C6H3-2,6- i Pr2) and the comparable cationic aminotropiniminate germanium(II) [(i Pr)2ATI]Ge+ have been isolated by Power and co-workers and Dias and Wang respectively, which confirms the high stability of such cationic derivatives of germanium(II) with supporting polydentate ligands. The structures of these compounds were determined. The structural features of the chloro-, the iodo- and the methyl compounds are quite similar (Figures 1- 3 respectively). In all these divalent species, the germanium center is at the apex of a distorted trigonal pyramid. Perusal of the germanium-ligand atom distances suggest that the ligand is essentially symmetrically bound to the germanium. The five ligand atoms are almost coplanar. The side views (Figure 4) of the three compounds show that the iodogermylene has the most planar C3N2 ring, whereas the methylgermylene has the greatest deviation from the planarity. Figure 1 : Solid state structure of L2 (Cl)Ge (ellipsoids are drawn at 50 % probability level). Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and bond angles (°). I. Composés du germanium et étain II stabilisés par le ligand ß-diiminate. 16 Figure 3 : Solid state structure of L2 (Me)Ge (ellipsoids are drawn at 50 % probability level). Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and bond angles (°)OMe Experimental and calculated (in parentheses) values of the distances of C(H) and Ge from the C3N2 plane (x and y, respectively) for L2(X)Ge. Chapitre I : Mise au point bibliographique 17 In all these compounds the average Ge-N bonds (~1.98 Å) are between Ge-N donor-acceptor bonds (2.05-2.11 Å) and covalent Ge(II)-N bonds (1.87-1.89 Å). The ClGeN angles are all close to 90°. The germanium-halogen lengths are about 10 % longer than the average of previously observed germanium-halogen distances in various dicoordinated germanium(II) and germanium(IV) compounds.84-50 This impressive margin reflects probably a halide medium-strongly bound to the germanium centres L 2Ge¼ X. To investigate the peculiar role that the ß-diketiminato ligand and the substituents play in these compounds, we have also analyzed their electronic structure by density functional theory (table 2) and by experimental UV photoelectron spectroscopy. Table 2 : Experimental ionization potentials, K-S energies and the nature of molecular orbitals for L2GeCl and L2GeI I. Composés du germanium et étain II stabilisés par le ligand ß-diiminate. 18 The He(I) and He(II) photoelectron spectra of the iodo- and chlorocompounds are reported in Figure 5. The first ionization potentials at 7.4 and 7.9 eV, for the iodo compound for example, correspond (as for the chloro compound) to the molecular orbitals having a strong participation of the germanium and the halogen lone pairs in interaction with the p (p3L) orbital of the ligand. The significant decrease of the intensity of the two bands corresponding at the higher energies (8.2 and 8.9 eV) on the He(II) irradiation is unambiguously indicative of the participation of the iodine lone pair orbitals. Concerning the ionizations of the lone pairs on the halogen atoms, it is noteworthy that they need lower energies for L²GeCl[9.7 eV (nCl)] and L²GeI [8.9, 8.2 eV(nI)] (Table 2) than for the corresponding dihalogermanium(II) compounds GeX2 88,89 (GeCl2 : 12.69 (a1, s + Cl), 12.58 (b2, p + Cl), 11.70 (a2, p – Cl), 11.44 (b1, s – Cl), aver. 12.10 eV; GeI2 : 10.62 (a1, s + I), 10.62 (b2, p + I), 9.83 (a2, p – I), 9.50 (b1, s – I), aver. 10.14 eV). This is indicative of more charged halogen atoms in the heteroleptic compounds L 2 (X)Ge than in dihalides GeX2.
Introduction Générale |