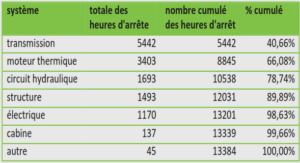
Attribuer les cas humains d’infections d’origine alimentaire à des sources spécifiques
Introduction
Pathogens commonly transmitted to humans through foods are responsible for a high burden of human illness and death worldwide. TheWorld Health Organization (WHO) estimates that 1.8 million children die each year from diarrhea, and much of the childhood diarrhea is caused by pathogens that are commonly acquired from contaminated food or water. Furthermore, even in developed countries up to one third of the population each year has an infection from a pathogen commonly transmitted through foods (WHO, 2005). Humans acquire these infections through a number of routes, including eating contaminated food, contact with live animals, and contact with a contaminated environment. Foodborne transmission is recognized as being responsible for a major proportion of these infections, and foodborne diseases may involve many different food sources and commodities. Several countries have implemented intervention programs during the last decades to prevent and control foodborne diseases, particularly foodborne zoonoses (Wegener et al., 2003; EFSA, 2006). However, precise measurement of the public health impact of such interventions has been difficult, in part because information on the attribution of the burden of foodborne diseases to specific sources is often insufficient. To prioritize appropriate food safety interventions, it is crucial to attribute the human disease burden of each foodborne infection to specific sources (FAO=WHO, 2006). A variety of general methods to attribute one or more foodborne diseases to specific sources has been developed, including microbiological approaches, epidemiological approaches, intervention studies, and expert elicitation approaches. Each of these general methods presents advantages and limitations, and the usefulness of each depends on the public health questions being addressed (Batz et al., 2005). Several groups are using attribution methods, but these often have different nomenclature and food categorization schemes. Defining scientific concepts and harmonizing terminology are essential for understanding and improving attribution methodologies and sharing knowledge across the scientific community. In this paper, we propose harmonized nomenclature; describe different approaches to human illness attribution; and discuss the advantages, limitations, and applicability of each approach in answering different questions along the farm-to-consumption continuum, while emphasizing that the choice of the attribution method will depend on the pathogen and the public health question being addressed.
General Approaches for Source Attribution
Microbiological approaches One general method for attribution of the human disease burden of foodborne infections to specific sources is ‘‘microbiological approaches.’’ Microbiological approaches for source attribution include the microbial subtyping approach and the comparative exposure assessment approach. Both approaches involve isolation of the pathogen from the various sources and from ill humans. The microbial subtyping approach requires a representative distribution of the subtypes of the pathogen in the different sources and humans, but does not depend on estimates of the prevalence of the subtypes in each source. The comparative exposure assessment approach requires estimates of the prevalence and concentration of the pathogen in each of the sources of exposure. Microbial subtyping approach. The microbial subtyping approach involves characterization of isolates of a specific pathogen by phenotypic and=or genotypic subtyping methods (e.g., serotyping, phage typing, antimicrobial susceptibility testing, pulsed-field gel electrophoresis, sequence-based subtyping). The principle is to compare the subtypes of isolates from different sources (e.g., animals, food) with those isolated from humans. The microbial subtyping approach is enabled by the identification of strong associations between some of the dominant subtypes and a specific reservoir or source, providing a heterogeneous distribution of subtypes among the sources. As a first step, subtypes isolated exclusively or almost exclusively from one source are regarded as ‘‘indicator subtypes,’’ and the human infections caused by each indicator subtype are assigned (attributed) to that specific source. The relationship between the relative occurrence (i.e., proportion of positive samples or positive isolates) of each indicator subtype in the source and the incidence of human infections caused by that indicator subtype is then determined. Finally, human infections caused by subtypes found in several sources are assigned to specific sources proportional to the occurrence of the indicator subtypes. The application of this approach assumes that the distribution of subtypes in the collection of isolates in each source used in the attribution exercise is similar to the true distribution of subtypes in each source. Because the microbial subtyping approach utilizes a collection of temporally and spatially related isolates from various sources, it is facilitated by an integrated foodborne disease surveillance program that is focused on the collection of isolates from the major food animal reservoirs of foodborne diseases. There have been several applications of the microbial subtyping approach for Salmonella source attribution (e.g., Van Pelt et al., 1999; Sarwari et al., 2001). The most advanced application of the microbial subtyping approach for Salmonella was developed in Denmark (Hald et al., 2004). Using data from the integrated Danish Salmonella surveillance program, a mathematical model was developed to quantify the contribution of each of the major food animal sources to human Salmonella infections. The ‘‘Danish Salmonella source account’’ model attributes domestically acquired laboratory-confirmed human Salmonella infections caused by different Salmonella subtypes (serotypes and phage types) as a function of the prevalence of these subtypes in animal and food sources and the amount of each food source consumed, using a Bayesian framework with Markov chain Monte Carlo simulation (Gilks et al., 1996). This microbial subtyping approach has proved to be a valuable tool in focusing food safety interventions to the appropriate animal reservoir in Denmark and provides an example of potential synergy between quantitative risk assessment and public health surveillance (Hald et al., 2004). Another example of the microbial subtyping approach is the use of multilocus sequence typing (MLST) of Campylobacter jejuni isolates from foods and humans, being applied in the United Kingdom (Dingle et al., 2002) and New Zealand (French, 2007). In this microbial subtyping approach, MLST is used to identify lineages in bacterial populations by indexing the variation present in seven housekeeping genes located in various parts of the chromosome (Dingle et al., 2001). With the development of novel analysis tools such as the ClonalFrame algorithm (Didelot and Falush, 2007), MLST has been used to identify clonal complexes associated with different isolation sources that, in some instances, correspond to different host species. As an increasing number of isolates, including isolates from various sources, are added to the MLST database (accessible on the internet), there will be increased precision in the attribution of human infections to host sources (McCarthy et al., 2007). Recently, MLST data were utilized to attribute the sources of human C. jejuni infections in New Zealand using two microbial subtyping models, the ClonalFrame algorithm and the Danish Salmonella source account; both models gave similar results (French, 2007)
Comparative exposure assessment approach
The principle of the comparative exposure assessment approach is to determine the relative importance of the known transmission routes by estimating the human exposure to that pathogen via each route. The comparative exposure assessment approach requires, for each known transmission route, information on the prevalence and dose of the pathogen in the source, the changes of the prevalence and quantity of the pathogen throughout the transmission chain, and the frequency at which humans are exposed by that route. These data provide an estimate of the exposure dose for each transmission route. The exposure doses are compared and the human disease burden (e.g., the observed laboratory-confirmed infections or estimated total number of infections) caused by the specific pathogen is partitioned to each of the various transmission routes, proportionally to the size of the exposure dose. The estimates of exposure dose for each transmission route can be combined with a dose–response model to predict the number of infections from each route, similar to what is done in traditional microbial risk assessments. The comparative exposure assessment approach for source attribution differs from traditional risk assessment in its objective and level of detail. A risk assessment typically aims at describing the complex dynamics of a pathogen in a single food commodity during food processing, and predicting the relative public-health effect of different interventions strategies—alone and in combination. In contrast, the comparative exposure assessment aims at partitioning the observed (or predicted) human disease burden to all known transmission routes, including various foods, direct contact with live animals, and environmental exposures. For this purpose, the various transmission routes are modeled in a more simplified and less detailed way that represents only the main steps in the transmission pathway.