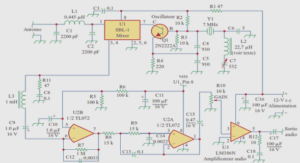
Amines synthesis via the Combination of the Wohl-Ziegler Reaction with Xanthate Chemistry
Carbon radicals stabilized by a phthalimido group possesses a small but nevertheless significant allylic character, which allow the efficient intermolecular radical addition of phthalimido-substituted xanthates to various unactivated alkenes. Although we have applied this feature to the synthesis of numerous phthalimide protected amines, it was important to expand the pool of obtaining xanthates to members not readily available by the previous methods. We were therefore intrigued by the bromination of 3-1 to form bromide 3-2 via the classical Wohl-Ziegler allylic bromination process. Displacement of bromine by xanthate group would afford the corresponding xanthate 3-3, which could then be employed in the typical radical additions to alkenes. This potentially flexible and general route for the construction of complex amines is generalized in Scheme 3.1.In view of the large pool of commercially available or easily accessible primary amines, success of the Wohl-Ziegler reaction in one case would considerably expand this approach to complex amines.
Bromination of N-phthalimide protected amines via the Wohl-Ziegler reaction
The Wohl-Ziegler reaction
In 1942, Ziegler developed a bromination process by using N-bromosuccinimide as a convenient brominating agent. Several years later Karrer found that the addition of a small amount of dibenzoyl peroxide significantly increased the reaction rate and the scope of this reaction was greatly extended. 85 Quickly chemists recognized that this reaction proceeded by a free radical chain process. Nowadays, the Wohl–Ziegler reaction has been defined as a reaction between an allylic or benzylic substrate with N-bromosuccinimide (NBS) under radical initiating conditions which can provide the corresponding allylic or benzylic bromide (Scheme 3.2)As shown in the scheme 3.3, heating a solution of AIBN releases nitrogen gas and leads to the formation of two tert-butyronitrile radicals. These radicals readily react with the small amount of Br2 present in the NBS to form a bromine atom. Light or heat may be used to initiate the reaction instead of AIBN or other initiators. In the following propagation step, the bromine atom abstracts an allylic hydrogen forming a stable allylic radical and HBr. This latter reacts immediately with NBS to maintain the low concentration of Br2 which plays an essential role in sustaining the chain process.Since a high concentration of Br2 would lead to the bromination of the double bond, a low concentration of Br2 istherefore critical for an efficient bromination.
The Wohl-Ziegler reaction in organic synthesis
Numerous brominations have been reported over the past decades, using the Wohl-Ziegler. The following studies demonstrate the wide applicability of this reaction. In the synthesis of oseltamivir, an anti-influenza neuramidase inhibitor, Corey and co-workers constructed the 1,3-cyclohexadiene intermediate via a Wohl-Ziegler bromination and elimination (Scheme 3.4). 87 A 95% yield of the brominated product attests to the efficiency that can be attained by this reaction. Harvey and co-workers applied the Wohl-Ziegler bromination in the course of the total synthesis of aigialomycin D (Scheme 3.5). 88 The benzylic bromide JEH-1 as a key intermediate was synthesized via a Wohl-Ziegler reaction. Instead of AIBN, benzoyl peroxide was used as an initiator in this reaction. Despite many applications of Wohl-Ziegler reaction in organic synthesis, some unsolved problems have somewhat limited the further applications of this reaction. Carbon tetrachloride is the most commonly used solvent in the Wohl-Ziegler reaction, but the toxic and ozone-depleting properties of carbon tetrachloride have made it less available and encouraged chemists to find alternative solvents. One solvent free Wohl-Ziegler reaction was developed by Rahman and co-workers as shown in Scheme 3.6. Using this solid-solid Wohl-Ziegler reaction, the bromination of diquinoline was completed in high yield.
Bromination of N-phthalimide protected amines based on the Wohl-Ziegler reaction
The application of the phthalimido as a protecting group to assist the synthesis of amines is extensively documented in the literature. In 1898, Sachs prepared N-bromomethylphthalimide from N-methylphthalimide by using bromine as the brominating agent; however, under the same conditions, N-ethylphthalimide gave only N-tribromoethylphthalimide. 90 In 1954, Zaugg successfully obtained N-(2-bromoethyl)-phthalimide in high yield from N-ethylphthalimide via Wohl-Ziegler synthetic method, which was the first bromination of N-alkylphthalimide via Wohl-Ziegler synthesis.91 In 1989, Easton and co-workers examined the bromination of a wide range of amino acid derivatives including N-phthalimide protected amino acid by using N-bromosuccinimide as the brominating agent.92 By comparing the rates of bromination, they discovered how different substituents influenced the stability of the radical intermediates generated from the protected amine substrates.93 Therefore, this study inspired us to synthesize novel phthalimide protected amine xanthates via the Wohl-Ziegler reaction. Furthermore,they described the bromination of phthalimide protected α-amino acid CJE-1 was quite inefficient, and required 48 h to achieve 50% conversion; however, the bromination of β-amino acid CJE-2 was completed with high efficiency in 84% yield (Scheme 3.7). A plausible explanation was then proposed. As described in Scheme 3.7, the steric effect arising from the interaction of the methoxycarbonyl and phthalimidyl group would destroy its planar configuration resulting in less efficient delocalization of the unpaired electron.