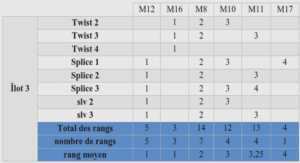
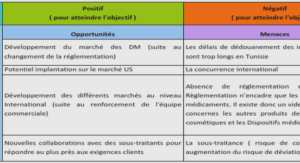
CYCLIC ALKYL(AMINO)CARBENE LIGAND FOR RUTHENIUM OLEFIN METATHESIS CATALYSTS
cyclic alkyl(amino)carbene CAAC [RuCl2(CAAC)(=CH-OiPr-C6H4)] C81. The structure and bonding parameters of complex C81 have been determined by X-ray diffraction and are compared with catalysts C65 and C67 reported previously by Grubbs. These ruthenium-based complexes are efficient catalysts in the ethenolysis of methyl oleate where high selectivity is required. Our new catalyst C81 displays higher activity than C65 and C67. Indeed, noticeable differences in catalytic activity were observed when the substituents on the carbene ligand were changed. The activity increased with the electron donor ability of the substituents. Olefin metathesis is a carbon-carbon bond forming reaction that is widely used in petrochemical, polymer, and specialty chemical industries1,2. Since the development of Grubbs catalysts which possess high functional group tolerance, significant current effort is focused on the modification of the ligand environment of ruthenium catalysts in order to produce new metathesis catalysts with improved stability, activity and selectivity. The replacement of tricyclohexylphosphine of C5 with N-heterocyclic carbene (NHC) gives rise to the more active and thermal stable complex C93, known as second-generation Grubbs As demonstrated in Chapter one, NHC ligands are generally considered as more -donor ligand than PCy3 (page 50). Indeed, the evaluation of C5 and C9 in the ethenolysis of methyl
oleate (Chapter II, page 143) have shown that NHC-complex C9 offers significantly enhanced activity relative to C5 in optimised conditions. Whereas the first-generation PCy3 based catalyst C5 was very selective toward ethenolysis products (1-decene and methyl-9- decenoate), NHC complexes C9 and C22 also catalyzed secondary metathesis as self- metathesis and isomerization process, which results in moderate overall reaction selectivity. We postulate that ligands which may have at once close steric environment to tricylcohexylphosphine to inhibit the self metathesis reaction, together with excellent -donor ability to provide a more stable ruthenacyclobutane during the catalytic cycle should afford tremendous precatalysts for the ethenolysis of methyl oleate. We thus decided to investigate the use of cyclic alkyl(amino)carbenes (CAACs) owing to their excellent steric and electronic properties7, as we have seen page 56. The steric environment of CAACs differs significantly from that of tertiary phosphines or NHCs. The exchange of an electronegative amine substituent in NHCs by the strong -donor carbon makes CAACs particularly electron-rich8. In addition, the presence of a quaternary carbon atom in -position to the carbene center offers the possibility of constructing ligands featuring different types of steric environments. Bertand and co-workers have shown that CAACs can compete with NHC as ligands for transition metal-based catalysts8. There was only one report on their use as ligands for ruthenium olefin metathesis when we began this study9. In the first part, we report the different synthetic approaches for CAACs ligands. Then, we describe their introduction on ruthenium metal center and our first results in the ethenolysis of methyl oleate.
56). The method used to prepare the precursor of acyclic alkyl(amino)carbenes, the alkylation of the corresponding enamines being limited10, Bertrand thus proposed a new synthetic approach to prepare CAAC11. The choice of R1 and R2 substituents is virtually unlimited (except that R1 and R2 cannot be H). The first CAAC L2 was obtained from imine L14 which can be synthesized from 2,6- diisopropylaniline and 2-methylpropanal. Deprotonation of L14 with lithium diisopropylamide (LDA) afforded the aza-allyle anion, which readily induces the ring opening of 1,2-epoxy-2-methylpropane leading to L15. The cyclic aldiminium salt L16 was obtained from L15 by reaction with trifluoromethanesulfonic anhydride (TfOTf) at -78°C. Finally, deprotonation of L16 with LDA gives rise to the carbene L2 as a white solid which is stable at ambient temperature. In this synthesis, the preparation of the nitrogen-containing heterocycles is tedious and time consuming, moreover 1,2-epoxy-2-methylpropane is difficult to obtain. Nitrogen-containing heterocycles are often obtained by intramolecular hydroamination of alkenes12, in which the nitrogen-carbon bond is formed by addition of an amine to an olefin. Hartwig and Schlummer developed an acid-catalyzed intramolecular hydroamination for aminoalkenes bearing